Accepted 27 August 2020
Available Online 2 September 2020
- DOI
- https://doi.org/10.2991/mathi.k.200829.001
- Keywords
- 3D printing
polyvinyl alcohol
paracetamol tablets
fused deposition modelling
sustained release - Copyright
- © 2020 The Authors. Published by Atlantis Press B.V.
- Open Access
- This is an open access article distributed under the CC BY-NC 4.0 license (http://creativecommons.org/licenses/by-nc/4.0/).
1. INTRODUCTION
Three-dimensional Printing (3DP) is an additive manufacturing process that can rapidly render 3D objects from computer aided design software by successively depositing polymeric materials layer by layer [1]. A range of different 3DP technologies are in current use, with the most prevalent methodology using Fused Deposition Modelling (FDM) technology due to a synergetic combination that merges economic and accuracy/resolution factors [2]. In 2015, Spritam®, a solid pharmaceutical formulation which contains the anti-epileptic drug levetiracetam, became the first 3D printed medicine approved by the US Food and Drug Administration leading to a raised interest in this type of technology so to revolutionise healthcare in the area of personalised medicines [3].
Paracetamol, a Class III Biopharmaceutics Classification System drug (low permeability, high solubility), is a well-known analgesic and antipyretic drug that currently available in different pharmaceutical dosage forms including tablets, suspensions and solutions. Tablets are recurrently the most common accessible paracetamol containing formulation and are typically produced using the direct powder compression methodology or granulation, if segregation of powder constituents is a problem [4,5]. However, these manufacturing processes exhibit limitations when compared to the extrusion method of 3DP. The most imperative limitation of the granulation method is lower Active Pharmaceutical Ingredient (API) loading resulting in higher final product total weight and material loss during processing [6], making 3DP an appealing alternative method for tableting manufacturing.
Few studies have investigated the production of paracetamol formulations using FDM printing [7,8]. Numerous studies have loaded drugs onto polymer filaments. Polyvinyl Alcohol (PVA), frequently utilised in coatings for tablets, is a commonly used filament and impregnation of the drug is achieved by soaking the filament in a highly saturated drug solution. This method results in low drug loading (<2% w/w) because of slow drug diffusion into polymer [9,10]. The use of hot melt extrusion has been reviewed as an alternative method by Tan to increase drug loading of API onto filaments [11]. However, to date there are no direct comparisons of the release profiles of 3D printed paracetamol tablets and commercial tablets. PVA is one of the most widely used water-soluble synthetic polymers and was selected because of its excellent thermoplasticity and it is the only commercially extruded polymer filament that would dissolve in vivo [9,12]. Herein we demonstrate the preparation of 3D printed paracetamol tablets using PVA, a biocompatible polymeric construct, and compare the drug release dissolution profile of the API with commercially available paracetamol containing tablets.
2. MATERIALS AND METHODS
2.1. Materials
Paracetamol USP grade, methanol (for HPLC, ≥99.9%) and potassium phosphate monobasic (KH2PO4) were purchased from Sigma-Aldrich Ltd, Gillingham, UK. PVA was purchased from 3D FilaPrint, Southend-on-Sea, UK. Milli-Q water (resistivity 18.2 MΩ cm) was used to produce all solutions. Paracetamol tablets were purchased from a pharmaceutical retailer (Boots, Nottingham, UK) with the following specifications: Paracetamol 500 mg Tablets (Batch No. 109T, Exp. Date. 08/2020, M&A Pharmachem Ltd, Bolton, UK).
2.2. Methods
2.2.1. Preparation of paracetamol-loaded PVA filament
Polyvinyl alcohol filament was cut into small pieces and milled using a Krups F20342 grinder (KRUPS, Windsor, UK) and sieved through a 1000 µm mesh. The milled PVA (100 g) and paracetamol powder (100 g) were combined using a mortar and pestle until a homogenous mixture was produced. The mixture was then extruded using a single-screw filament extruder (Wellzoom desktop extruder filament B, Shanghai, China) to obtain a drug loaded filament (extrusion temperature: 200 °C; nozzle diameter: 3 mm; screw speed: 25 rpm). The extruded filament was stored in a vacuum desiccator (Nalgene®, Rochester, USA) and protected from light until printing. The drug-loading of the filament was determined by UV analysis.
2.2.2. Additive manufactured paracetamol-loaded tablets printing specifications
Tablets were fabricated using the aforementioned PVA drug-loaded filaments and a commercial fused-deposition modelling 3D printer, Ultimaker 2 (Ultimaker, Geldermalsen, Netherlands). Tablets were designed using the freeware web-based application Tinkercad (Autodesk, San Rafael, USA) software, exported as a Standard Tessellation Language file and uploaded to the 3D printer software, Cura (Ultimaker). The 3D geometry of the tablet was cylindrical (12.83 mm diameter × 4.10 mm height). The fill density was set at 150% in order to produce solid dosage forms of high density. The printer settings that were found to produce the best tablets for PVA drug-loaded filament were of fine resolution, nozzle temperature (200 °C), nozzle diameter (0.4 mm), speed while extruding (30 mm/s), speed while travelling (30 mm/s), material flow (150%), number of shells (2), layer height (0.10 mm) and build plate temperature (60 °C). No supports or rafts were utilised in the printed model.
2.2.3. UV analysis and quantification of paracetamol samples
Paracetamol analysis was conducted using a Genova Plus UV/Visible spectrophotometer (Cole-Parmer, Staffordshire, UK). The UV readings were acquired at a wavelength of 242 nm. A stock solution (24 μg/mL) was prepared from a known amount of paracetamol dissolved in a 2% methanolic solution. The stock solution was diluted accordingly to prepare various concentrations of paracetamol ranging from 1.2 to 24 μg/mL. A calibration curve was constructed with a linear relationship between concentration and absorbance with regression coefficient values (r2) of greater than 0.999.
2.2.4. Testing conditions for tablet dissolution
Tablet dissolution profiles for both commercially available and 3D printed formulations (n = 3) were obtained using a USP II apparatus (Caleva 10ST, Erweka GmbH, Germany). The formulations were placed in 900 mL of a KH2PO4 buffer solution (pH 5.8, 0.032M) for 60 min to simulate the primary environment of the small intestine compartment. Paddle speed for USP II equipment was fixed at 50 rpm and the sampling conducted at 37 ± 0.5°C under sink conditions. Aliquots from the dissolution media (1 mL) were taken at standardised time intervals and the volume replaced with fresh KH2PO4 buffer solution (pH 5.8, 0.032M). Dissolution media samples were diluted to fit the calibration curve and paracetamol concentration was determined using the aforementioned UV analysis methodology.
3. RESULTS AND DISCUSSION
Herein we show how PVA was used for the printing of tablets as a main polymeric filler. PVA is a well-known excipient used in the pharmaceutical industry acting as a binder in solid drug formulation [13]. This biocompatible polymer presents various desirable qualities for medicine manufacture such as high-water solubility and tensile modulus (3500 MPa) [14]. Unlike polycaprolactone, another biocompatible polymer with lower water solubility and extrusion temperature, PVA was shown to have positive FDM 3DP capabilities which intend to recreate other important tablet properties [i.e. hardness, friability, disintegration (shown in the Supplementary Materials) and dissolution] [14].
Paracetamol loaded PVA filament (3 mm) was successfully manufactured by blending a mixture of the API and PVA using hot melt extrusion. Tablets were initially fabricated using neat PVA filament to simulate the optimum conditions for 3DP (Figure 1A). Drug loaded printlets were similarly produced in cylindrical shape (Figure 1B). The drug loading of the filament was 39%. This was lower than the theoretical value as a result of adhesion of the fine API powder to the walls of the barrel of the HME during the extrusion process [15]. The tablets showed similar values to the filament indicating that no degradation took place during the printing process. Tablets also showed high uniformity in size and weight (mean dimensions: 12.93 mm diameter × 4.08 mm height, mean weight: 1.2459 g) which rendered a concise method of manufacture. Erokhin et al. [16] have demonstrated that higher infill densities of 3D scaffolds reduce the shrinkage of polymers when printing thus resulting in marginally larger diameters in comparison to the original designs. The height of the 3D printed construct shown here was comparable to the designed tablet, also demonstrating good adhesion between the printed layers. The dose of paracetamol in the 3D printed tablet was 484.6 ± 18 mg in comparison to the commercial tablets that contained 500 ± 2 mg.
Printlets (A) PVA and (B) paracetamol-loaded PVA.
The dissolution profiles of commercial and PVA-loaded 3D printed paracetamol tablets were collected over a period of 60 min as shown in Figure 2. The results gathered for the dissolution of the commercially available formulation met the guidelines set by the British Pharmacopeia [17], whereby at least 80% of the API is released within 30 min.
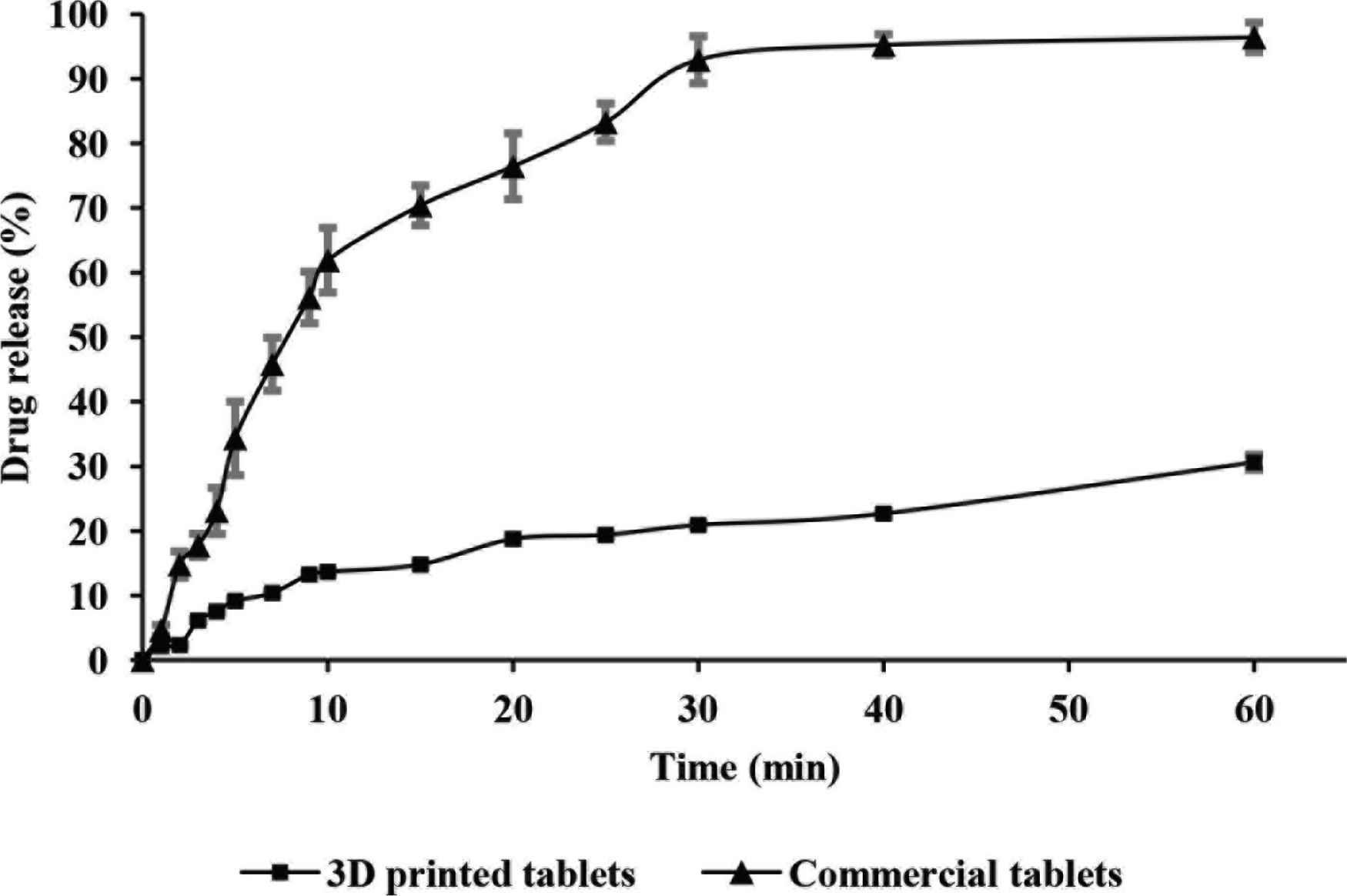
Dissolution profiles for commercial (▲) and PVA-loaded 3D printed (■) tablets (n = 3, Mean ± SD).
In comparison, the dissolution profile presented for the PVA-loaded 3D printed paracetamol tablets did not comply with the aforementioned guidelines since only 20.91 ± 0.32% of drug content was released from the formulation within 30 min and 30.61 ± 1.18% upon experimental completion (Figure 2). In light of these results, further investigations were conducted to ascertain a more accurate paracetamol release profile. Consequently, PVA-loaded 3D printed paracetamol tablets were left in dissolution testing conditions for a total period of 24 h with results showing an extended release profile of the drug (63% drug release). Chai et al. [18] also demonstrated a similar trend in drug release rates for commercial and 3D printed domperidone tablets, where 3% of the API was released from the 3D printed formulation after 15 min, with a sustained release profile emerging after 12 h, although the drug loading was significantly lower.
4. CONCLUSION
Here we successfully demonstrate the 3DP of paracetamol tablets using PVA-loaded filament with a 100% infill density. The dissolution studies of the API from 3D printed paracetamol tablets showed a sustained release profile in comparison to commercial paracetamol tablets. This work exhibits the potential FDM printing brings to the manufacture of medicines, however high extrusion temperatures for PVA printing can be seen as a possible disadvantage and the use of alternative polymers may permit printing at lower temperatures to avoid degradation of the drug. Future studies will involve the potential use of different polymers and APIs with maintained biocompatibility and expected extend release profiles, respectively.
CONFLICTS OF INTEREST
The authors declare they have no conflicts of interest.
AUTHORS’ CONTRIBUTION
Conceptualization, BCS and BP; methodology, MA; analysis, MA, BCS and BP; writing – original draft preparation, BCS and BP; writing – review and editing, BCS and BP; visualization, BCS and BP; supervision, BCS and BP.
FUNDING
We thank
SUPPLEMENTARY MATERIALS
Supplementary data related to this article can be found at
REFERENCES
Cite This Article

TY - JOUR AU - Maryam Asmari AU - Bruno C. Sil AU - Bhaven Patel PY - 2020 DA - 2020/09/02 TI - An Undergraduate Study for the Comparison of Dissolution Profiles using 3D Printed PVA and Commercial Paracetamol Tablets JO - Materials Highlights SP - 22 EP - 25 VL - 1 IS - 1-2 SN - 2666-4933 UR - https://doi.org/10.2991/mathi.k.200829.001 DO - https://doi.org/10.2991/mathi.k.200829.001 ID - Asmari2020 ER -








